2023 Innovations in Regulatory Science Summit
Accelerating Healthcare Innovation Through a Regulatory Lens
Sunday, January 8, 2023 | 9 am to 4 pm Pacific Time








About This Event
The UCSF-Stanford Center of Excellence in Regulatory Science and Innovation (UCSF-Stanford CERSI) is pleased to present the fourth annual Innovations in Regulatory Science Summit, a gathering of leaders in the academia, industry, and regulatory sectors to discuss the role of regulatory science in medical product development.
The 2023 CERSI Innovations in Regulatory Science Summit will have a major focus on emerging issues in regulatory science including diversity in clinical trials, FDA/CMS decision making, and global drug development. In addition, we will be bringing back our exciting panel from 2022 featuring former FDA Commissioners discussing the latest issues facing the Agency.
The goal of the summit is to stimulate discussion across sectors and to critically consider lessons learned and scientific issues that need addressing to expeditiously and safely advance the development, approval, and monitoring of medical products.
Panel Discussions:
- Disparities in Clinical Trial Enrollment: What Will it Take to Close the Gap?
- Cross Agency Synergy to Accelerate Access to Safe and Effective Medical Products
- Building a Global Regulatory Vision for Product and Drug Development: Challenges and Opportunities
- FDA Chiefs Chat
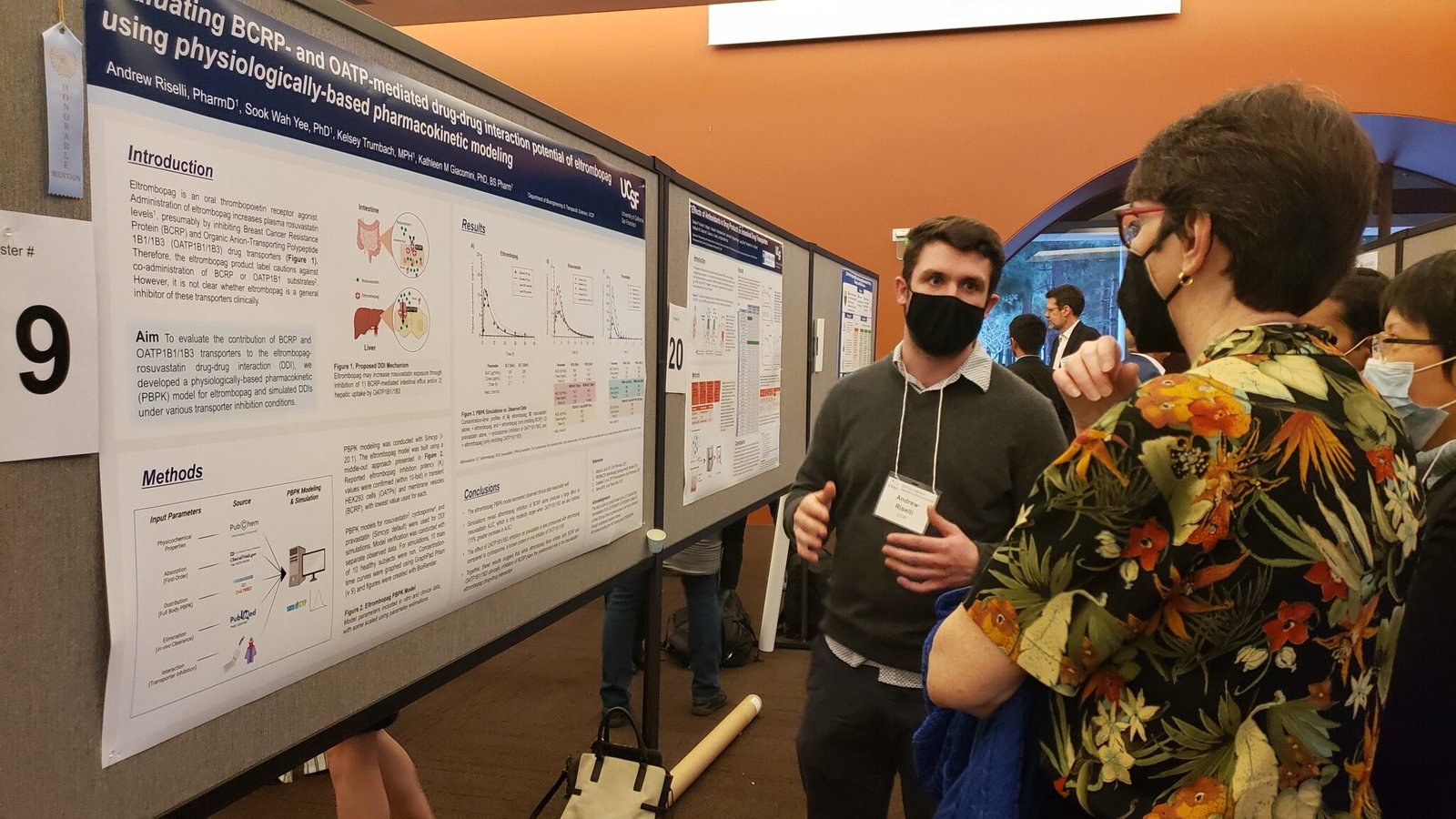
Call for Posters:
The 2023 Innovations in Regulatory Science Summit Planning Committee invites abstract submissions to be presented at our in-person poster session in San Francisco, CA on Sunday, January 8, 2023. We invite all trainees and researchers to share their regulatory science research at our summit.
Agenda
Times shown in Pacific time (UTC-8)
8:15 - 9:00 am
Breakfast & Sign-In
9:00 - 9:10 am
Welcome
Kathy Giacomini, PhD, BSPharm – Co-PI, UCSF-Stanford CERSI
Kuldev Singh, MD, MPH – Professor of Ophthalmology at Stanford University
9:10 - 9:15 am
Opening Remarks
Sam Hawgood, MBBS – Chancellor, University of California San Francisco (UCSF)
9:15 - 9:35 am
Year in Review
Robert Califf, MD – FDA Commissioner, 2016-2017, 2022-present
9:35 - 10:35 am
Panel 1 – Disparities in Clinical Trial Enrollment: What Will it Take to Close the Gap?
Moderators:
Laura Esserman, MD, MBA – Professor of Surgery and Radiology at UCSF
Patrizia Cavazzoni, MD – Director, Center for Drug Evaluation and Research (CDER) at FDA
Panelists:
Michael Drake, MD – President, University of California
Congresswoman Anna Eshoo – Chair of the Health Subcommittee in the US House of Representatives
Ricki Fairley, MBA – Chief Executive Officer at Touch, The Black Breast Cancer Alliance
Sohail Rao, MD, MA, DPhil – Founding President & Chief Executive Officer at DHR Health Institute for Research & Development
Ramona Sequeira, MBA – President, Global Portfolio Division at Takeda Pharmaceutical
10:35 - 10:55 am
Break
10:55 - 11:55 am
Panel 2 – Cross-Agency Synergy to Accelerate Access to Safe and Effective Medical Products
Moderators:
Kuldev Singh, MD, MPH – Professor of Ophthalmology at Stanford University
Mark McClellan, MD, PhD – Professor of Business, Medicine, and Policy at Duke University
Panelists:
Rena Conti, PhD – Associate Research Director of Biopharma & Public Policy at the Boston University Institute for Health System Innovation & Policy
Lee Fleisher, MD – Chief Medical Officer and Director of the Center for Clinical Standards and Quality at the Centers for Medicare and Medicaid Services (CMS)
Mathai Mammen, MD, PhD – Executive Vice President, Pharmaceuticals, Research & Development at Johnson & Johnson
Jeff Shuren, MD, JD – Director, Center for Devices and Radiological Health (CDRH) at FDA
11:55 am - 12:55 pm
Panel 3 – Building a Global Regulatory Vision for Product and Drug Development: Challenges and Opportunities
Moderators:
Kathy Giacomini, PhD, BSPharm– Co-PI, UCSF-Stanford CERSI
Dan Hartman, MD – Director, Integrated Development, Global Health at Bill & Melinda Gates Foundation
Panelists:
Moji Christianah Adeyeye, PhD, FAS – Director-General at the National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria
Emer Cooke, MBA – Executive Director of the European Medicines Agency (EMA)
Frank Gupton, PhD – Floyd D. Gottwald, Jr. Chair in Pharmaceutical Engineering and Chair, Professor, Department of Chemical and Life Science Engineering at Virginia Commonwealth University
Peter Marks, MD, PhD – Director, Center for Biologics Evaluation and Research (CBER) at FDA
Jacques Mascaro, PhD, MBA – Senior Vice President, Global Head of Oncology Regulatory Science and Strategy at AstraZeneca
12:55 - 1:40 pm
Lunch
1:40 - 2:40 pm
Panel 4 – FDA Chiefs Chat
Moderators:
Andy Plump, MD, PhD – President, Research and Development at Takeda Pharmaceutical
Janet Woodcock, MD – FDA Principal Deputy Commissioner
Panelists:
Robert Califf, MD – FDA Commissioner, 2016-2017, 2022-present
Scott Gottlieb, MD – FDA Commissioner, 2017-2019
Margaret (Peggy) A. Hamburg, MD – FDA Commissioner, 2009-2015
Mark McClellan, MD, PhD – FDA Commissioner, 2002-2004
2:40 - 2:55 pm
Closing Remarks
Janet Woodcock, MD – FDA Principal Deputy Commissioner
Laura Esserman, MD, MBA – Professor of Surgery and Radiology at UCSF
2:55 - 4:00 pm
Poster Session and Networking
Kathy Giacomini, PhD, BSPharm – Co-PI, UCSF-Stanford CERSI
Tina Morrison, PhD – Director, Office of Regulatory Science and Innovation (ORSI) at FDA
4:00 pm
Adjournment
Confirmed Speakers, Panelists, and Moderators
Moji Christianah Adeyeye
National Agency for Food and Drug Administration and Control (NAFDAC) in Nigeria
Robert Califf
U.S. Food and Drug Administration
Patrizia Cavazzoni
U.S. Food and Drug Administration
Rena Conti
Boston University
Emer Cooke
European Medicines Agency
Michael Drake
University of California
Anna Eshoo
U.S. House of Representatives
Laura Esserman
University of California, San Francisco
Ricki Fairley
Touch, The Black Breast Cancer Alliance
Lee Fleisher
Centers for Medicare and Medicaid Services
Kathy Giacomini
University of California, San Francisco
Scott Gottlieb
Former FDA Commissioner
Frank Gupton
Virginia Commonwealth University
Margaret A. Hamburg
Former FDA Commissioner
Dan Hartman
Bill & Melinda Gates Foundation
Sam Hawgood
University of California, San Francisco
Mathai Mammen
Johnson & Johnson
Peter Marks
U.S. Food and Drug Administration
Jacques Mascaro
AstraZeneca
Mark McClellan
Duke University
Tina Morrison
U.S. Food and Drug Administration
Andy Plump
Takeda Pharmaceutical
Sohail Rao
DHR Health Institute for Research & Development
Ramona Sequeira
Takeda Pharmaceutical
Jeff Shuren
U.S. Food and Drug Administration
Kuldev Singh
Stanford University
Janet Woodcock
U.S. Food and Drug Administration
About UCSF-Stanford CERSI
The UCSF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) is a joint effort among members of a world-class team of highly collaborative scientists at the University of California San Francisco, Stanford University, and the U.S. Food and Drug Administration (FDA). The two academic institutions and the FDA work together on projects that promote regulatory science—including innovative research, education, and scientific exchange—in partnership with foundations and commercial entities interested in the development of FDA-approved medical products. The center collaborates, in particular, with the pharmaceutical, biotechnology, and high-tech industries of the San Francisco Bay Area and the West Coast.
Contact
For information or questions about this event, please contact the UCSF-Stanford Center of Excellence in Regulatory Science and Innovation at info@ucsfstanfordcersi.org.
Sponsors
PLATINUM SPONSORS

GOLD SPONSORS


SILVER SPONSORS



BRONZE SPONSORS

